The Forgotten Microbiome
Compared to us, microbes seem primitive and simple. We humans are large and vast in numbers. Our innovations can be seen from space, which makes it seem like we rule the world. This assumption would be incorrect as microbial life, specifically single-celled microbes, are the oldest life forms on earth.
We are prime real estate for the microbial community. If all homosapiens went extinct, the microbial world would not be bothered. On the other hand, if it weren’t for our relationship with these microbes, humans would likely not be able to exist as we do today. These beings are just as, if not more, evolved than humans and much more adaptive to our changing environment than we are. It was once thought that the human body’s microbes outnumber own cells within our own bodies by 10 to 1. However, the ratio is actually thought to be about 1:1, meaning humans are made up of half microbes and half human cells.
We are all simply evolutionary products of a long line of organisms who figured out how to develop and maintain a symbiotic relationship with the microbial world (Sonnenburg 2016). Understanding the relationship between gut health and our behaviors is the essential first step to understanding ourselves, our communities, our environment and our planet. In other words: our collective health.
Knowing this, it should be no surprise that the vast number of bacteria living in our guts influence many parts of our health such as immune regulation, digestive assistance, and nutrient assimilation. However, as we learn more about the microbiome, we are continuously baffled by exactly how much influence these bacteria have, not just on our physical bodies, but on our daily mentality, moods, and behaviors. The scientific community has even come to lovingly call our gut’s microbiome a “forgotten organ” or “second brain” due to it’s increasingly clear impact on our health.
Unfortunately, a lot of our current knowledge on the microbiome has been overlooked, drawn out, and not completely understood. The existence of microscopic organisms was officially discovered by Robert Hooke and Antoni van Leeuwenhoek in the journal Micrographia in 1665, when the microfungus, Mucor, was depicted. This was the very first time a microorganism was illustrated (Howard 2005). Since then, the microbial world has sent human scientists onto a wild goose chase of contradictory evidence, correlations without causation, alarming gut-brain axis studies, and even misinformation by corrupt entities.
The confusion around such an important discovery like the microbiome could be due to the many injustices perpetrated on microbial life throughout the history of scientific research, such as the overlooking of the work of scientists. Linda R. Hegstrand and Roberta Jean Hine may be the most important example of why historical context is vital. In 1986 the pair quite literally discovered and proved that microbes influence brain chemistry. This was the first time anyone proved the link between the microbiota and the brain. A study like this has incredibly important implications; however, due to the vast and ignorant oversight of this discovery, this study’s total citations to date are at 3, while more than 500 studies, research papers, and journals have cited the false information that continuously claims that the term microbiome was ‘coined’ by Nobel laureate and microbiologist Joshua Lederberg in 2001 (Prescot 2017).
It may seem like a small oversight, but in the Human Microbiome Journal, Susan Prescot explains
“It matters because... the consequences can lead to ‘scientific myth’. At the extreme end this could influence policy and practice. However, even at its most fundamental level, factual science referencing is a matter of ethics. Providing credit where credit is due. When an erroneous attribution is afforded to one researcher, especially a term or quotation in common parlance, the individual or group of individuals – the legitimate source – will remain in the scientific shadows”
Unfortunately, we may very well be seeing consequences of our lack of understanding and the spread of misinformation today.
Dr. Alan C Logan describes microbial dysbiosis as a global health issue. In 2016 he published an article explaining
“The implications of the microbiome extend to virtually every branch of medicine, biopsychosocial and environmental sciences. Similarly, the impact of changes at the immune-microbiota interface 1 are directly relevant to broader discussions concerning rapid urbanization, antibiotics, agricultural practices, environmental pollutants, highly processed foods/beverages and socioeconomic disparities.”
Current statistics tell the story: in the USA alone, 60 to 70 million people are affected by digestive disease. 6 in 10 adults in the US have a chronic disease and 4 in 10 adults in the US have two or more chronic diseases. (CDC)
Using the Microbiome as a Client Specific Map
As a nutrition professional, the goal is to improve our client’s health as quickly and safely as possible, while keeping true to the client’s own comfort, pace, and biochemical individuality. Today, there's an endless amount of combinations of chemical, dietary, lifestyle, environmental, social, and viral risk factors to consider when assessing client health. To add to the chaos, modern misinformation is the most prevalent it’s ever been. Health and wellness professionals often do a good amount of digging into a client's life to explain and uncover the underlying factors contributing to their current health situation. It is also common to see clients struggle to find motivation for making healthful changes and building healthy habits. Can we better support our clients by observing and learning from behavioral changes they make themselves when we see gut health improves? If we make alterations to a client’s gut health by adding microbial diversity and improving gut hygiene, we will then see the client make independent changes in other areas of their health as a result of them having a happier and healthier gut?
Today, the study of the microbiome is made possible thanks to a powerful scientific species of rodent known as “Gnotobiotic rodents''.
Gnotobiotic rodents are lab rats or mice who have never been exposed to any microbial life. These “germ-free” rodents have pre-determined microbiota. Every aspect of these rodent's lives are sterile from birth: when they’re delivered by Cesarean section the pregnancy sac is dipped into a light bleach solution and from then on the rodents are raised in a completely sterile environment. The air, water, food, and friends they interact with are all sterile and the only microbiota present in these animals are ones that have been introduced by the scientists studying them. Studies using these germ-free environments and animals are reliable. When using these methods to analyze a certain microbial strain scientists can thus have complete confidence that microbial influence is completely controlled. (Turnbaugh 2009)
It’s important to note that these animals are not seen as a "control group" since the lack of microbes is unnatural on our planet. Instead, these animals are considered a "laboratory meta-organism" since the specific physiology of these animals can only exist in the laboratory world (Turnbaugh 2009). Instead of acting as a control, these animals are used to set a basis for what the body does on its own basic biological mechanical level.
What are the known impacts of gut bacteria and human health?
What exactly are the impacts gut bacteria have on human health? A common and frustrating trend to note among research into the microbiota and gut health is that while correlation and causation are apparent, the exact mechanisms of the bacteria in relation to our bodies - how they work in and interact with our bodies - are complex, multifactorial, and not well understood. Health and food scientists Yu-Jie Zhang, Sha Li, Ren-You Gan, Tong Zhou, Dong-Ping Xu, and Hua-Bin Li, published a meta-analysis in 2015 conveniently called “Impacts of Gut Bacteria on Human Health and Diseases” which in great detail summarizes and discusses the role and potential mechanisms of gut bacteria in human health and diseases.
Essential Takeaways
The completely germ-free rodent presented with both structural and functional abnormalities and reductions such as “reduced vascularity, digestive enzyme activity, muscle wall thickness, cytokine production and serum immunoglobulin levels, smaller Peyer’s patches and fewer intraepithelial lymphocytes” as well as a reduced adversity to danger.
This seems to indicate that, since germ-free conditions are not normal, it is obvious that from birth the microbiota is supporting not only the tissues GI but many vital parts of the body's function. When these rodents were compared to the “humanized” rodents, which are rodents inoculated with human microbiota, it’s clear that gut bacteria benefit the host in those same areas. As well as regulating gut motility, producing vitamins, transforming bile acid and steroids, metabolizing xenobiotic substances, absorbing minerals, and activating and destroying toxins, genotoxins, and mutagens (Zhang 2015).
Interestingly enough, when similar trials were done to study obesity, it seems microbiota were solely responsible for weight gain from a standard American diet since when germ-free mice were fed that same diet, they did not gain any weight. The meta-analysis also provides extensive research linking gut-bacteria and its relationship to inflammatory gut issues, obesity, diabetes, liver disease, heart disease and cancer.
Notable mechanics are:
"Reciprocal interaction between commensal gut bacteria and the host may induce allergies and IBD. Overly aggressive Th-1 mediated cytokine response to commensal bacteria may be the pathogen of chronic intestinal inflammation. In addition, disorders in bacterial recognition by macrophages are strongly related to pathogenesis of IBD. Furthermore, IBD could result from an abnormal immune response against the commensal microbiota in a genetically susceptible host.”(Zhang 2015)
“Numbers of lactobacilli were significantly lower during the active phase of Ulcerative colitis, and denaturing gradient gel electrophoresis analysis suggested that... (certain bacterial strains) were present in remission, but not during active inflammation.”(Zhang 2015)
"Normal gut bacteria play an important role in diet-induced obesity, because germ-free mice have been reported to be thinner and did not become adipose when subjected to high-fat diet [66]....Gut bacteria could also affect obesity by promoting chronic inflammatory status.”(Zhang 2015)
“Gut bacteria have a direct link with the risk of cardiovascular diseases. They form trimethylamine (TMA) from dietary choline after its conversion into TMA N-oxide in the liver."(Zhang 2015)
Does the microbiome influence our behavior and mood? If so, how do these microbes interact with the rest of the body to do so?
From this review, it’s obvious that manipulations of the gut flora in gnotobiotic animals and limited human trials have been proven to induce physical changes. So what about mental changes?
In 2011, a research group at Mcmaster University set out to answer this very question in an experiment. Working with two different groups of gnotobiotic animals, one group were anxious and timid natured mice they named “The Woody Allens” while the other group, named “The Robert Benignis”, were a much more confident and extroverted bunch. To determine this, the scientists placed the mice on a raised platform and recorded the length of time it took for them to step down.
The Woody Allens took much longer as a group than the Robert Benignis. However, when the two groups were inoculated with a fecal transplant of the opposing group, the results switched as well. The once confident Robert Benignis took well over a minute to step down from the platform in the second trial, while the Woody Allens reduced their time stepping off by over a minute. Not only did the two groups' behaviors change drastically, but both groups also experienced measurable changes in brain chemistry following the transplant.(Sonnenburg 2016)
The exact mechanisms of this behavior change is unclear, but researchers found that the transplants affected levels of a protein called BDNF. This protein has been associated with diseases such as depression, schizophrenia, and OCD, and low levels of BDNF are indicated in depressive behaviors. (Sonnenburg 2016) Many have investigated how exactly gut microbes interact with the rest of our bodies.
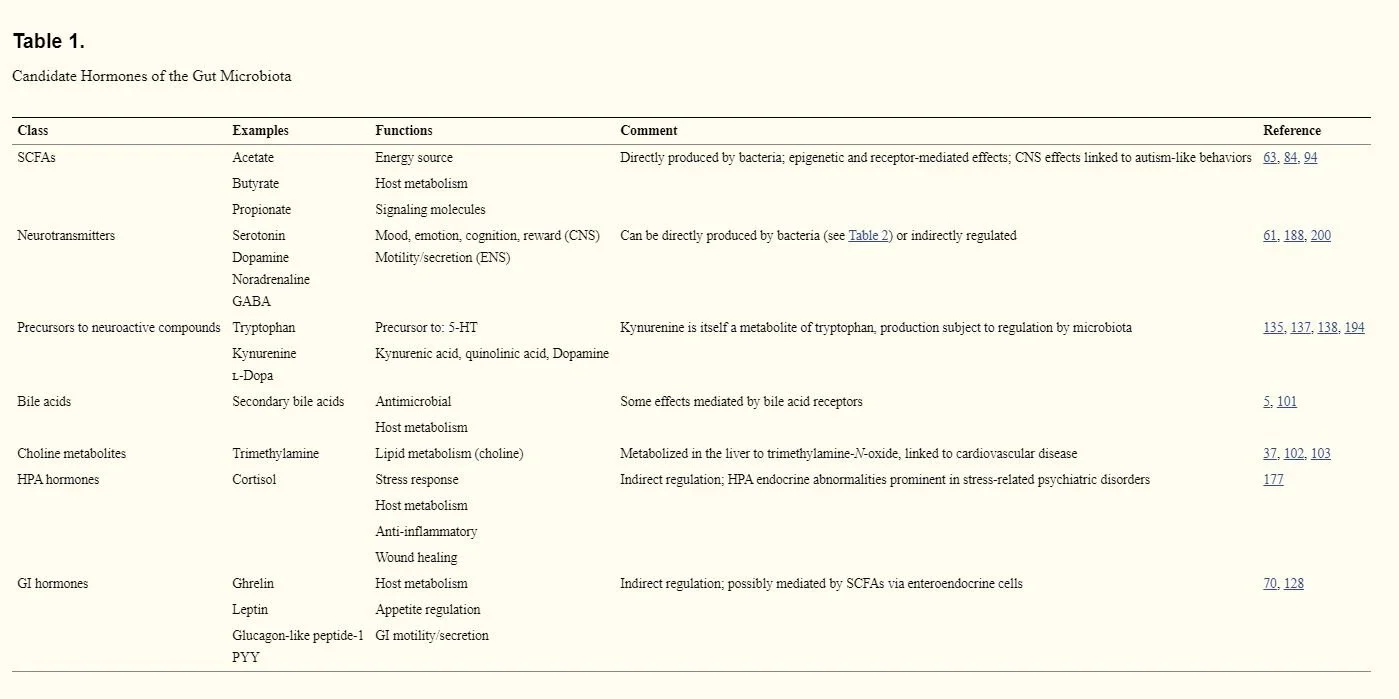
In 2014, scientist Gerard Clarke, along with his team, conducted a detailed observational study comparing the microbiome to the endocrine system called “Gut Microbiota: The Neglected Endocrine Organ” in which, through this comparative lens, they discovered the microbiota produces numerous chemicals of a hormonal nature that are released into the bloodstream (Table 1.)
“ Microbial metabolites such as SCFAs with signaling functions are secreted into the gut lumen, transported across the epithelial barrier, and transported to the effector organs, including the brain, via the bloodstream. The gut microbiota is also capable of producing or releasing neurotransmitters such as serotonin or regulating the availability of precursors such as tryptophan.” -Clarke 2014
This is also backed up by research done by Partice Cani and Claude Knauf who conducted an overview of the different interactions existing between microbial metabolites, endocrine and nervous routes in the body. (Cani 2016) Clarke’s study concluded that
“The biochemical complexity of the gut microbiota even exceeds that of the brain, and many of the hormones produced by the microbiota are also neurotransmitters within the central nervous system (CNS)...The gut microbiota performs a number of essential protective, structural, and metabolic functions for host health, including food processing, digestion of complex host-indigestible polysaccharides, pathogen displacement, and synthesis of vitamins. As well as a direct action on the gut mucosa and the enteric nervous system (ENS), the metabolic output of the gut microbiota gives it a reach well beyond the local GI compartment. Thus, considering the ability to influence the function of distal organs and systems, in many respects, the gut microbiota resembles an endocrine organ.” - Clarke 2014
What Constitutes as a “Healthy” Gut Microbiome?
We don’t currently have a clear understanding of what constitutes a “healthy” gut microbiome. However, a picture is emerging from many recent studies identifying particular bacterial strains and species that have been associated with health benefits and a “healthy” microbiome.
In 2013 “Richness of human gut microbiome correlates with metabolic markers” was published in “Nature”. This was an experimental study done on 123 non-obese and 292 obese individuals. They found that participants with a low bacterial “richness” or diversity were characterized by an increase in overall adiposity, insulin resistance and dyslipidemia, as well as a more pronounced inflammatory constitution and tended to gain more weight over time when compared with the participants with high bacterial “richness” or diversity (Le Chatelier, 2013).
While many studies support these claims and many experiments show similar findings, scientists at Oxford University warn that the relationship to diversity is much more complex than it seems. Katerina Johnson wrote
“Findings have notable implications for analysis of gut microbiome data, indicating that diversity may have a more complicated relationship with microbiome health and stability than often considered. Future studies should look beyond the classic diversity indices and seek to develop and apply novel methods for assessing microbiome composition and functioning.”
Understanding that a diverse microbiome is much more protective and functional than a less diverse one, but also taking into account that it is not the only factor upon which a healthy gut-flora relationship is built, scientists Jason Lloyd-Price, Galeb Abu-Ali and Curtis Huttenhower conducted a thorough review of factors that constitute “the healthy human microbiome.” They concluded that, in addition to a microbial richness and abundance, a healthy human microbiome will also have a functional core, and resilience stating,
“A necessary condition for a healthy microbiome is therefore the presence of an assemblage of microbial species that can carry out specific sets of biomolecular functions in each of the niche-specific biochemical environments across the body.” -Lloyd-Price 2016
A functional core suggests that the key to a healthy microbiome is one that includes specific combinations of bacteria that work well from the start and can mediate each other. For example, they state, children birthed through the vaginal canal will have a much more functional core than children birthed through C-section. Resilience, resistance, and stability is measured in this review by the amount of time it takes for the microbiome to recover to close to its original state following antibiotic treatment.(Lloyd-Price 2016)
Generally, a more resilient gut will be one with a functional community of microbiota who have regular access to microbiota accessible carbohydrates (MAC), a balanced healthy diet, and regular movement and exercise (Sonnenburg 2016). MACs are defined as the fibers that are accessible and able to be used and fermented by the gut flora. The fermentation of microbiota accessible carbohydrates by gut flora provides the human body with quality and available short chain fatty-acids (SCFAs). MACs are used instead of dietary fiber, since many staple “fiber foods” in the standard American diet are unable to be fermented by the gut biome and thus simply pass through our bodies.
Dietary Support
Until recently, plant foods, ferments, and high quality proteins were regularly circulating through the tube that is our digestive system. We've systemically suffocated the microbiome and forgotten how to feed and fertilize our gut gardens. As strong as the microbiome is, our gut is just as efficient and evolved. There's no room for freeloaders, but rather a team effort to process a meal takes place. All microbes feed on material that otherwise goes unused in the body, but they don't all have the same palette. Specific MACs nourish different strains of microbiota so diversifying your food will in turn diversify the gut flora. In this sense, Eating for Health (Bauman 2017) and eating for your microbes are one in the same.
|
Recommended Foods That Support Gut Health |
||
|
MACs |
Ferments and Probiotics |
Digestive Herbs |
|
The research indicated in the literature review supports the link between MACs from high plant fiber foods and health benefits reaped from the production of microbial SCFAs; however, there's no specific way to measure MACs so "the dietary fiber content serves as the best available approximation" (Sonnenburg 2016). Polyphenol rich foods like blueberries, dark chocolate, and red wine are not digested very efficiently by the human gut, but are able to be broken down and assimilated by our microbiome. |
Fermented and Probiotic foods are full of beneficial bacteria. It’s essential to reintroduce fresh and new strains of bacteria to the gut regularly to maintain diversity. Fermented foods are particularly useful since they usually contain an already diverse array of safe bacteria who are working well together in the fermentable environment, as opposed to a big dose of one of the FDA approved mono cultured strains found in many supplements. These supplements are simply tested to be proven to be safe for consumption, rather than tested for health benefits.(Sonnenburg 2016) |
Digestive herbs, specifically: Ginger, Turmeric, Black Pepper, and Long Pepper have been studied and proven to alter the microbiome. Each of these herbs when introduced to the gut, expanded and reduced different strains of bacteria, involving the bacteria with isoflavone biotransformation, bile acid conversion, and generation of cholesterol-derived compounds. (Peterson 2019) |
|
Examples: Leafy greens, legumes, apples, peaches, mangos, root and tuber vegetables, crunchy vegetables,and nuts and seeds. |
Examples: Kimchi, sauerkraut, yogurt, tempeh, miso, pickles, kombucha, and kefir. |
Examples: Curcuma longa, Zingiber officinale, Piper longum, and Piper nigrum |
|
Foods to Avoid That Deplete Gut Health |
|
|
Saturated Fats |
Many of the gnotobiotic experiments revealed that diets high in saturated animal fats are detrimental to microbial diversity. Bacteria who thrive on these saturated fats tend to be pathobionts. (Sonnenburg 2016) (Kirpich 2016) |
|
Red Meat |
Red meats contain the chemical L-carnitine which a few strains of microbes can convert into a compound called trimethylamine which when oxidized transforms into trimethylamine-oxide. High levels of trimethylamine-oxide are associated with the increased risk of cardiac events and stroke (Janeiro 2018). Limiting the consumption of red meat will reduce health risks. |
|
Gluten Containing foods |
Gluten is an allergen and not easy to digest. Cutting it out of the diet can lower chronic inflammation and give the gut time to heal and cool down. |
|
Sugary Foods and Refined Sugars |
Refined sugars are inflammatory, feed pathobiont bacteria, and can throw off blood sugar and pressure. Many refined sugars and sugar substitutes contain synthetic chemicals and additives that can disrupt the endocrine system as well as damage the actual gut bacteria’s DNA. (Harpaz 2018) |
|
Refined Empty Carbohydrates |
Empty carbohydrates don’t provide our gut flora with nutrients and can cause inflammation which disrupts the gut biome. (Bauman 2016) |
|
Stimulants |
Overconsumption of stimulants such as coffee, alcohol, and energy drinks disrupt the circadian rhythm, encourage candida overgrowth, and deplete nutrients.(Bauman 2016) |
Lifestyle Support
The four pillars of health in lifestyle are stress, sleep, community and environment. Looking at the relationship an individual has with these three lifestyle factors can tell us a lot about their current health (Bauman 2016). While diet is one of the most important modifying factors of the microbiota, the routes of communication between the microbiota and the body are slowly being unraveled and it turns out diet is not the only thing that alters our gut flora. Lifestyle factors have just as important of an effect as dietary changes do. This is especially true since a conventionally “unhealthy” lifestyle is usually accompanied by a conventionally unhealthy diet. So, what supports gut health and what depletes it?
|
Gut Flora Support for Lifestyle |
|
|
Exercise: |
Regular physical activity not only carries endless benefits to the body, but promotes the growth of beneficial gut bacteria such as Akkermansia, which has been indicated in the prevention of obesity and may play a role in metabolic health. These positive effects are not seen in individuals who are inactive or sedentary.(Clarke 2014) (Plovier 2016) |
|
Sleep: |
Disruption of the circadian rhythm and the effects of sleep deprivation seem to affect the gut microbiome by causing subtle changes. However, the exact mechanics and effect this has on health is unknown. |
|
Stress Management: |
Some ways to lower stress may include meditation, walking, getting a massage, spending time with friends or family, diffusing essential oils, decreasing caffeine intake, laughing, yoga, or having a pet. |
|
Gut Flora Depletors for Lifestyle |
|
|
Antibiotic Treatments: |
While they’re extremely effective in fighting the terrifying parasitic bacteria that live in this world, antibiotic treatments do not discriminate. All bacteria are harmed in the gut during antibiotic treatment and one single round has harmful effects on the gut short term. Long term harm has been observed after antibiotic treatment as well, most gut microbiomes do not return back to the levels of flora they had before the treatments. |
|
NSAIDS and Analgesic Drugs and Xenoestrogens: |
All have been shown to alter and harm gut bacteria as well as other essential areas of digestion (Bauman 2016). |
|
Tobacco Smoke: |
Smoking tobacco is addictive and has detrimental effects on the whole body when overconsumed. One study shows that regular smokers who stopped smoking saw an increase of diversity in gut flora after nine weeks.(Biedermann 2013) |
|
Stress: |
The effects of stress on the body are well researched and it’s established that long-term stress can have serious harmful effects on the body. In the gut, stress is shown to increase sensitivity and reduce blood flow. Some gnotobiotic studies have shown that different types of stress alter the gut flora in different ways. However the consensus is that stress exposure causes an increase in potentially harmful bacteria while reducing beneficial populations. (Palma 2015) (Bharwani 2015) |
Disclaimer & References:
Disclaimer: The information provided by Cherry & Pepper and Sasha Abiaad is intended for your general knowledge only and is not a substitute for medical advice or treatment for specific medical conditions. None of my services shall be used to diagnose or treat any health problem or disease. I cannot and do not provide medical advice. You should seek prompt medical care for any specific health issues and consult your physician before altering your diet. The information and recipes provided on this site and in my session plans should not be used in place of a consultation with your physician or other health care provider. I do not recommend the self-management of health problems. Should you have any healthcare-related questions, please consult your physician or other health care provider promptly. You should never disregard medical advice or delay in seeking it because of the information provided in this plan.
Bauman, E., & Friedlander, J (2016). Foundations of nutrition textbook- part 2. Penngrove, CA: Bauman College.
Bauman, E., & Friedlander, J. (2016) Therapeutic nutrition textbook-part 1. Penngrove, Ca: Bauman college.
Benedict, C., Vogel, H., Jonas, W., Woting, A., Blaut, M., Schürmann, A., & Cedernaes, J. (2016, October 24). Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5123208/
Bharwani A;Mian MF;Foster JA;Surette MG;Bienenstock J;Forsythe P; (n.d.). Structural & Functional Consequences of Chronic Psychosocial Stress on the Microbiome & Host. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26479188/
Biedermann, L., Zeitz, J., Mwinyi, J., Sutter-Minder, E., Rehman, A., Ott, S. J., … Rogler, G. (2013). Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3597605/
Cani, P. D. (2018, September 1). Human gut microbiome: hopes, threats and promises. Retrieved from https://gut.bmj.com/content/67/9/1716
CE;, S. A. E. C. N. (n.d.). Effect of Antimicrobial Agents on the Ecological Balance of Human Microflora. Retrieved from https://pubmed.ncbi.nlm.nih.gov/11871461/
Chatelier, E. L., Nielsen, T., Qin, J., Prifti, E., Hildebrand, F., Falony, G., … Pedersen, O. (2013, August 28). Richness of human gut microbiome correlates with metabolic markers. Retrieved from https://www.nature.com/articles/nature12506
Centers for Disease Control and Prevention. (2019, October 23). Chronic Diseases in America. Retrieved from https://www.cdc.gov/chronicdisease/resources/infographic/chronic-diseases.htm
Clarke SF;Murphy EF;O'Sullivan O;Lucey AJ;Humphreys M;Hogan A;Hayes P;O'Reilly M;Jeffery IB;Wood-Martin R;Kerins DM;Quigley E;Ross RP;O'Toole PW;Molloy MG;Falvey E;Shanahan F;Cotter PD; (n.d.). Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Retrieved from https://pubmed.ncbi.nlm.nih.gov/25021423/ DA;, D. L. H. S. S. M. L. R. (n.d.). The Pervasive Effects of an Antibiotic on the Human Gut Microbiota, as Revealed by Deep 16S rRNA Sequencing. Retrieved from https://pubmed.ncbi.nlm.nih.gov/19018661/
Daïen, C. I., Pinget, G. V., Tan, J. K., & Macia, L. (2017, May 12). Detrimental Impact of Microbiota-Accessible Carbohydrate-Deprived Diet on Gut and Immune Homeostasis: An Overview. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5427073/
De Palma G;Blennerhassett P;Lu J;Deng Y;Park AJ;Green W;Denou E;Silva MA;Santacruz A;Sanz Y;Surette MG;Verdu EF;Collins SM;Bercik P; (n.d.). Microbiota and Host Determinants of Behavioural Phenotype in Maternally Separated Mice. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26218677/
DeGruttola, A. K., Low, D., Mizoguchi, A., & Mizoguchi, E. (2016, May). Current Understanding of Dysbiosis in Disease in Human and Animal Models. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4838534/
Dinan, T. G., Stilling, R. M., Stanton, C., & Cryan, J. F. (2015, March 3). Collective unconscious: How gut microbes shape human behavior. Retrieved from https://www.sciencedirect.com/science/article/pii/S0022395615000655
DL;, P. S. L. W. G. L. A. C. K. (n.d.). Dysbiotic Drift and Biopsychosocial Medicine: How the Microbiome Links Personal, Public and Planetary Health. Retrieved from https://pubmed.ncbi.nlm.nih.gov/29743938/?from_term=Logan+AC+micro&from_pos=3
Eetemadi, A., Rai, N., Pereira, B. M. P., Kim, M., Schmitz, H., & Tagkopoulos, I. (2020, April 3). The Computational Diet: A Review of Computational Methods Across Diet, Microbiome, and Health. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7146706/
Gur, T. L., Worly, B. L., & Bailey, M. T. (2015, February 2). Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313583/
H;, G. (n.d.). The Remarkable Vision of Robert Hooke (1635-1703): First Observer of the Microbial World. Retrieved from https://pubmed.ncbi.nlm.nih.gov/15834198/ Janeiro, M. H., Ramírez, M. J., Milagro, F. I., Martínez, J. A., & Solas, M. (2018, October 1). Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6213249/
JF;, S. E. D. T. G. C. (n.d.). Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Retrieved from https://pubmed.ncbi.nlm.nih.gov/28768369/
Jumpertz R;Le DS;Turnbaugh PJ;Trinidad C;Bogardus C;Gordon JI;Krakoff J; (n.d.). Energy-balance Studies Reveal Associations Between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans. Retrieved from https://pubmed.ncbi.nlm.nih.gov/21543530/
Kirpich, I. A., Petrosino, J., Ajami, N., Feng, W., Wang, Y., Liu, Y., … McClain, C. J. (2016, March 21). Saturated and Unsaturated Dietary Fats Differentially Modulate Ethanol-Induced Changes in Gut Microbiome and Metabolome in a Mouse Model of Alcoholic Liver Disease. Retrieved from https://www.sciencedirect.com/science/article/pii/S0002944016000195
Microbiome: Should we diversify from diversity? (n.d.). Retrieved from https://www.tandfonline.com/doi/full/10.1080/19490976.2016.1241933
Milani, C., Duranti, S., Bottacini, F., Casey, E., Turroni, F., Mahony, J., … Ventura, M. (2017, November 8). The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5706746/
Mills, S., Lane, J. A., Smith, G. J., Grimaldi, K. A., Ross, R. P., & Stanton, C. (2019, June 27). Precision Nutrition and the Microbiome Part II: Potential Opportunities and Pathways to Commercialisation. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6683087/
Mutlu, E. A., Gillevet, P. M., Rangwala, H., Sikaroodi, M., Naqvi, A., Engen, P. A., … Keshavarzian, A. (2012, May 1). Colonic microbiome is altered in alcoholism. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3362077/
Peterson, T., C., Rodionov, A., D., Iablokov, N., S., … Meredith A. (2019, June 2). Prebiotic Potential of Culinary Spices Used to Support Digestion and Bioabsorption. Retrieved from https://www.hindawi.com/journals/ecam/2019/8973704/
Plovier H;Everard A;Druart C;Depommier C;Van Hul M;Geurts L;Chilloux J;Ottman N;Duparc T;Lichtenstein L;Myridakis A;Delzenne NM;Klievink J;Bhattacharjee A;van der Ark KC;Aalvink S;Martinez LO;Dumas ME;Maiter D;Loumaye A;Hermans MP;Thissen JP;Belzer C;de Vos WM;Cani PD; (n.d.). A Purified Membrane Protein From Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Retrieved from https://pubmed.ncbi.nlm.nih.gov/27892954/
Prescott, S. L. (2017, June 7). History of medicine: Origin of the term microbiome and why it matters. Retrieved from https://www.sciencedirect.com/science/article/pii/S245223171730012X#b0055
Rinninella E;Cintoni M;Raoul P;Lopetuso LR;Scaldaferri F;Pulcini G;Miggiano GAD;Gasbarrini A;Mele MC; (n.d.). Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Retrieved from https://pubmed.ncbi.nlm.nih.gov/31591348/?from_term=healthy+gut+changes&from_pos=2
Ruiz-Ojeda, F. J., Plaza-Díaz, J., Sáez-Lara, M. J., & Gil, A. (2019, January 1). Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6363527/
SL;, L. A. C. J. F. N. P. (n.d.). Immune-Microbiota Interactions: Dysbiosis as a Global Health Issue. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26768621/?from_term=Logan+AC+micro&from_pos=2
Sonnenburg, J., & Sonnenburg, E. (2016). The good gut: taking control of your weight, your mood, and your long-term health. Penguin Books.
Tap, J., Furet, J. P., Bensaada, M., Philippe, C., Roth, H., Rabot, S., … Leclerc, M. (2015, September 3). Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Retrieved from https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1111/1462-2920.13006
Turnbaugh, P. J., Ridaura, V. K., Faith, J. J., Rey, F. E., Knight, R., & Gordon, J. I. (2009, November 11). The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Retrieved from https://stm.sciencemag.org/content/1/6/6ra14?utm_source=feedburner&utm_medium=email&utm_cam paign=Feed:+IsagenixNewsroom+IsaFYI
Zhang, Y.-J., Li, S., Gan, R.-Y., Zhou, T., Xu, D.-P., & Li, H.-B. (2015, April 2). Impacts of gut bacteria on human health and diseases. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4425030/